WATER ACTIVITY (Aw)- An Essential Quality parameter for Non-Sterile Pharmaceutical Products:

SPOC SCIENTIFICS(Single. Point. Of. Contact) is reliable supplier for the WATER ACTIVITY instrument of different models from NOVASINA make, Switzerland. For more details, refer link: https://www.novasina.ch/lab/products-lab/
We know Non-sterile drug products are more susceptible to microbial growth during their shelf life stability. Because of this reason, most of the drug products are formulating /developing by using antimicrobial preservatives to protect the patients from these potential hazards.
In sometimes, there are some other product attributes also using to prevent microbial contamination include: low pH, low redox potential, reduced storage temperature, packaging that protects the product leads to reduced water activity level.Reduced water activity (Aw) will greatly assist in the prevention of microbial proliferation in pharmaceutical products. So, the Water Activity of a compound can be reduced majorly by:
- Temperature (lowering temp decreases Aw – i.e. storage conditions)
- Adding preservatives or antimicrobial agents
- Reducing the amount of water in product
Water activity, or Aw, is a measure of available water, applied to the non-sterile pharmaceutical drug product as a critical physical attribute that determines whether the product will support the growth of microorganisms. Given the knowledge of the minimum water activity for the growth of bacteria, yeast and mold, the microbial stability of a drug product can be determined. This knowledge can be used to set risk-based microbial specification for release and stability testing programs for different
dosage forms.
The determination of the water activity of non-sterile pharmaceutical dosage forms aids in the decisions relating to the following aspects:
- Optimizing product formulations to improve antimicrobial effectiveness of preservative systems,
- Reducing the degradation of active pharmaceutical ingredients within product formulations susceptible to chemical hydrolysis.
- Reducing the susceptibility of formulations (especially liquids, ointments, lotions, and creams) to microbial contamination, and
- Providing a tool for the rationale for reducing the frequency of microbial limit testing and screening for objectionable microorganisms for product release and stability testing using methods contained in the general test chapter “Microbial Enumeration Tests <61> and Tests for Specified Microorganisms <62>”.
Water activity ranges from 1.00 for pure water to 0.00 for a dry material. As microorganisms that would be found in pharmaceutical products require a water activity of at least 0.75 for microbial growth, the proliferation of microorganisms within a drug product can becontrolled by the inherent low water activity and/or reducing the water activity of the drug product. For example, oral liquids with a water activity of greater than 0.95, if unpreserved, would support microbial growth, especially bacteria, while a topical ointment with a water activity of 0.58 would not support any microbial growth. Since microorganisms need water within a pharmaceutical product to proliferate, water activity and not water content is a better measure of the free water, in contrast to bound water that microbial cells require formetabolic activity and osmotic regulation.
The most common pharmaceutical dosage form (>80%), compressed tablets, typically have water activities in the range of 0.3-0.4, hence,if stored in packaging that excludes moisture, such as high-density polyethylene bottles with heat induction seals and screw-top lids, will not support microbiological growth during the drug product shelf life.

Growth range at water activity & Relative humidity (%ERH) for various classes of micro-organisms.
From Regulations perspective:
Testing procedures and acceptance criteria for drug release programs are outlined in ICH Q6A. Instructions on best methods for determining microbiological attributes are found in Decision Trees #6 and #8. In both decision trees, the need for microbial limits testing is based on whether the product is inherently “dry” enough to not support microbial growth.
The assumption in the pharmaceutical industry is that this dryness can be established using moisture content, or amount of water in a product, usually through a Karl Fischer analysis. However, since the work of Scott in the 1950’s, it has been well established that it is water activity, or the energy of water, that actually determines whether or not microorganisms can access the water in a system. It means, No correlation between moisture content and microbial growth. Therefore, the “dryness” referenced in the decision trees of ICH Q6A should be measured using water activity.
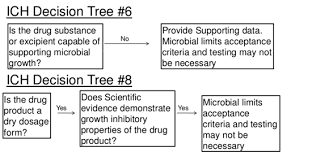
Water activity should also be considered and utilized when constructing a stability protocol using ICH Q1A. An information from USP Chapter, also provides further scientific evidence that water activity should necessarily be part of any risk-based drug release quality program to ensure microbial safety and product quality.
Water Activity Vs Microbial Growth:
Water activity is the best index for microbial growth. A product may contain a relatively large percentage of moisture, but if the water is chemically “bound” to solutes, such as API & excipients, the water is biologically unavailable for microbial growth. Every microorganism has a limiting water activity below which it cannot grow. No direct relationship exists for moisture content and microbial growth.
Water Activity in QbD:
A drug manufacturer that decides to develop their nonsterile drug release program based on QbD principles needs to identify their CQA’s. If they determine that microbial attributes are one of their CQA’s, they will need to setup one or several CPP’s that will describe the microbial safety of their product. Currently, most pharmaceutical companies will follow USP <61>and conduct microbial limits release testing. However, the purpose of both ICH and QbD is to develop risk based, science backed programs. Minimum water activity limits for microbial growth are well established in the scientific literature as well as in USP. Consequently, especially for solid dosage products, water activity makes more sense as a CPP for establishing microbial safety than microbial testing because it is cheaper, faster, and scientifically
sound.
Furthermore, it is more reliable than microbial testing since it establishes the safety of an entire batch while microbial testing only assures the integrity of the sample tested.
Increasing/ difference in water activity levels between product components indicates:
Water activity is not only useful as a microbial CPP, but also has merit as a CPP for other CQA’s such as product integrity, API stability, and dissolution. Differences in water activity levels between components or a component and the environmental humidity is a driving force for moisture migration. If an API is combined with an excipient or coating system at different water activities, water can migrate to the API making it more susceptible to degradation. Water leaving a coating due to water activity differences will cause cracking while water movement into the coating will make the coating sticky. Many excipient/drug combinations are amorphous systems. As water activity increases, the plasticizing effect of water increases resulting in moisture induced phase changes. These phase changes are equivalent to a glass transition that can lead to crystallization, which changes dissolution and increases degradation of Active Pharmaceutical Ingredients
(API). A critical water activity for the induction of glass transition can be established using a moisture sorption isotherm analysis. This critical water activity can then be used as a CPP for dissolution and API stability.
Water activity is a useful measure of microbial stability that can be successfully used in product development and microbial risk assessments to support specification setting and testing decisions for both product release and stability studies.
Both USP 922 and USP 1112 highlight the potential applications for water activity. These include stability control, microbial risk prevention, optimized formulation, reduced caking and clumping, and moisture migration control, which all have significant product improvement advantages. The resulting key benefits are: Less consumer complaints, greater confidence, higher production output with consequently better products for the consumer, and greater profits for the manufacturer. Conclusively, water activity is a powerful, and often essential, quality parameter for pharmaceutical products.
Action:
Any requirements about Water Activity’ instrument, please contact us at : email: spocsupplies@gmail.com,info@spocscientifics.com,
Whatsapp / Call us: +919030178155, +919030168155

